Introduction to suppressive T cells
Regulatory T cells (Treg) in suppressive T cells are a subpopulation of T cells characterized by the expression of Foxp3, CD25, and CD4 as cell phenotype markers.
Introduction to suppressive T cells
Regulatory T cells (Treg cells) are an important subset of T cells that regulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune diseases. Treg cells have immunosuppressive properties and typically inhibit or downregulate the induction and proliferation of effector T cells.
There are two main types of Treg cells in humans: the main population of Foxp3+ Treg cells are produced in the thymus and are called tTreg cells. Under certain conditions of peripheral sites or in vitro exposure to TGFβ, Foxp3- conventional T cells (Tconv cells) can produce an unknown percentage of Treg cells, called pTreg/iTreg cells. There are multiple forms of regulatory T cells, with the most well-known being those that express CD4, CD25, and FOXP3 (CD4+CD25+ regulatory T cells). Other types of Treg cells include Tr1 cells, Th3 cells, and CD8+CD28- regulatory T cells.”
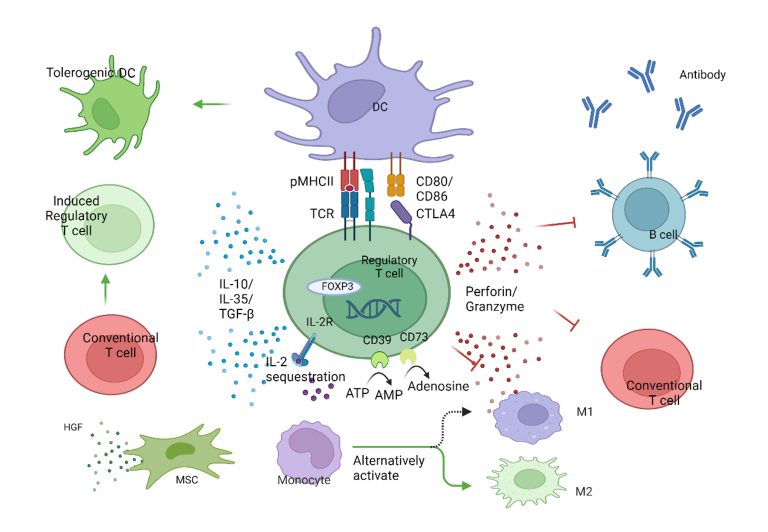
Treg cells suppress pro-inflammatory effects and limit excessive immune response
The main function of Treg is to directly suppress the immune cell function, infiltration, and inflammation. At the cellular level, Treg acts by inhibiting upstream APC cells (DC) and downstream effector cells (Tconv, macrophages, B, NK cells, etc.). In brief, Treg mainly exerts its inhibitory function through various cell contact-dependent and independent mechanisms.
- CTLA-4-dependent Tconv suppression, LAG-3-mediated DC maturation inhibition.
- By expressing high-affinity IL2R, it deprives other T cells of IL-2.
- Through the extracellular enzymes CD39 and CD73, it produces immunosuppressive nucleotides adenosine.
- Secretion of anti-inflammatory cytokines IL-10, TGF-β, and IL-35.
- Induces apoptosis of inflammatory cells through the secretion of granules/enzymes.
- Polarizes macrophages from M1 to M2 type.
- Induces the activity of immune-inhibitory enzyme IDO in DCs.
- Regulatory T cells (Tregs) coordinate with each subpopulation and together play an immunoregulatory role in maintaining homeostasis in the body. Regulatory T cells play a crucial role in controlling and preventing autoimmune diseases. Mesenchymal stromal cells (MSCs) can regulate immune responses in various diseases and exhibit potent regenerative and reparative abilities similar to stem cells. Compared to injecting Tregs or MSCs alone, the combined infusion of Tregs and MSCs can rescue mild liver fibrosis. The reason may be that Tregs can increase the expression of hepatocyte growth factor (HGF) and the differentiation ability of MSCs. The two major cytokines expressed by Tregs are TGF-β and IL-10, and experiments have shown that Tregs can regulate the TGF-β-IDO signaling pathway to enhance the function of MSCs. Both MSCs and MSC-conditioned medium (MSC-CM) can inhibit necrotic inflammation and fibrogenesis in the model, but the latter method is more effective. In this environment, Tregs and Th2 cells are activated, and the number of Th17 cells decreases.
Promoting organizational repair
In recent years, it has been discovered that Tregs also have a role in promoting tissue healing. Tregs can produce various growth factors in different tissues to facilitate the repair of different tissues, for example:
- Keratinocyte Growth Factor (KGF) in the lungs
- Alkaline Fibroblast Growth Factor 2 in the intestines
- Nerve Growth Factor in the spinal cord
- Neurotrophic Factor in the spinal cord
- Insulin-like Growth Factor 1 in the heart and retina
- In addition, Tregs also use AREG to promote tissue repair in multiple organs. AREG is an epidermal growth factor (EGF) family protein produced by various cell types. Burzyn et al. first demonstrated that Tregs accumulate at muscle injury sites. Tregs inhibit the production of IFN-γ by NK and effector T cells, macrophage activation, and inflammation in injured muscles. Additionally, Tregs can also directly affect muscle cells. Overall, Tregs and AREG play a crucial role in muscle repair. Tregs themselves also play an important role in the repair of central nervous system (CNS) tissues. Tregs can promote myelin regeneration after acute CNS damage by promoting oligodendrocyte differentiation. In a traumatic brain injury model, IL-2 can increase the residence of Tregs in the CNS, reduce neuroinflammation, and prevent nerve damage during the recovery process. Considering the different roles of Tregs in repairing and controlling tissue damage in the above situations, we can infer that the development of Tregs has the potential to develop new therapies for promoting healing of damaged muscles, neurons, and myelin sheaths, providing effective treatment for clinical difficult conditions such as amyotrophic lateral sclerosis, multiple sclerosis, opticospinal encephalomyelitis, etc.
Treg mechanism diagram